Introduction
Stomata are essential microscopic structures on the surface of plant leaves and stems, facilitating critical physiological processes such as gas exchange and transpiration (Bergmann & Sack, 2007). These pores are bordered by a pair of specialized guard cells, whose turgor changes regulate the opening and closing of the stomata, thus balancing carbon dioxide uptake for photosynthesis with minimizing water loss (Chen, L., 2023). This regulation is fundamental to plant growth, development, and adaptation to environmental stresses (Houbaert et al., 2018).
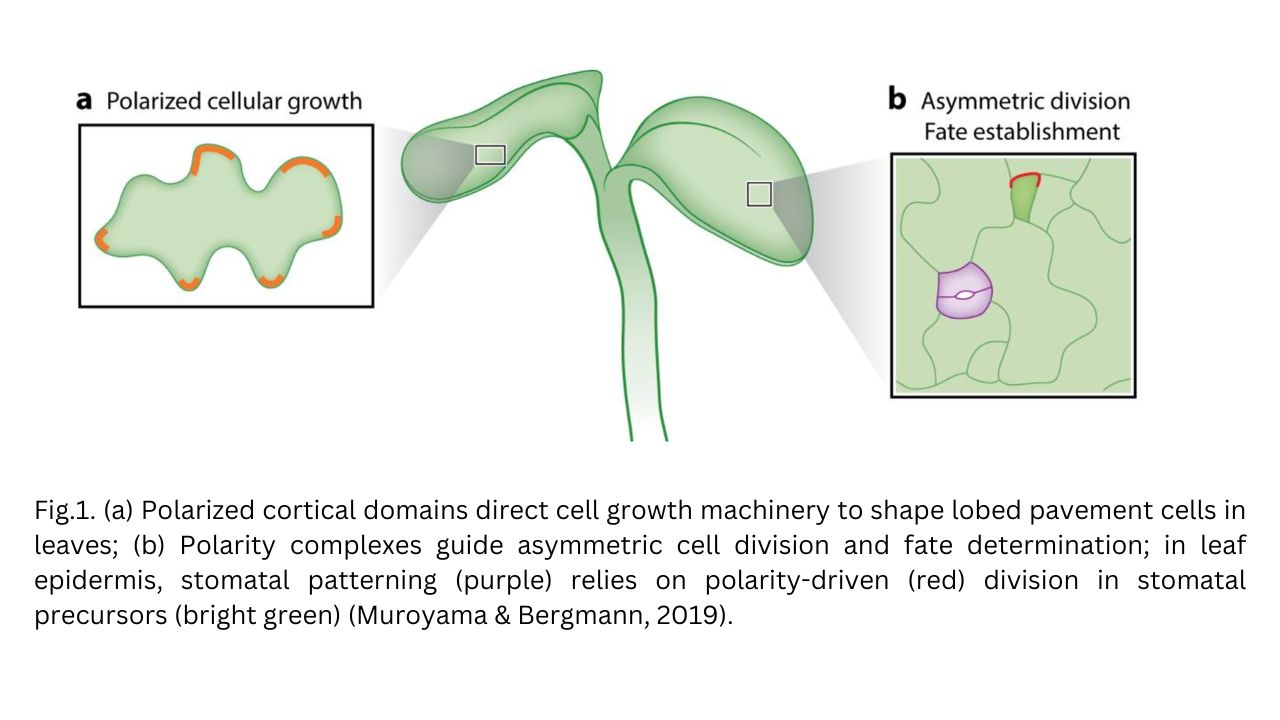
The development of stomata is a complex and highly regulated process involving a series of asymmetric and symmetric cell divisions within the stomatal lineage (Muroyama & Bergmann, 2019). Asymmetric divisions produce daughter cells with distinct fates, ensuring proper stomatal patterning and spacing, critical for their functionality (Bergmann & Sack, 2007).
Cellular polarity plays a vital role in this process by establishing and maintaining the directional distribution of molecular components within cells (Sampathkumar et al., 2014). This polarity regulation ensures that specific proteins and signals are localized to particular regions of the cell, guiding division orientation and developmental outcomes (Zhang et al., 2023).
Protein kinases, particularly the AGC family of serine/threonine kinases, have emerged as central players in regulating cellular polarity and signaling pathways in plants (Muroyama & Bergmann, 2019). Among these, AGC1 kinases are implicated in various cellular processes, including polarity establishment, vesicle trafficking, and cytoskeletal dynamics (Sampathkumar et al., 2014).
Recent studies suggest that AGC1 kinases interact with key polarity proteins such as BASL (BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE) and POLAR, coordinating the asymmetric divisions essential for stomatal development (Houbaert et al., 2018). However, despite their importance, the precise molecular mechanisms underlying AGC1 kinase function in these processes remain poorly understood.
This project aims to elucidate the role of AGC1 kinases in stomatal development by investigating their influence on polarity regulation and associated signaling pathways. Specifically, the study seeks to analyze expression patterns, protein interactions, and downstream targets of AGC1 kinases to uncover how these kinases contribute to the establishment of polarity and the coordination of asymmetric cell divisions. The findings will advance our understanding of stomatal development and provide broader insights into the regulatory networks governing plant growth and adaptation.
Also Study About: Signal Transduction in Plant
Literature Review
Stomatal Development in Plants
Stomatal development is a highly regulated process essential for plant physiology, as it governs gas exchange and water loss (Bergmann & Sack, 2007). This process begins with the asymmetric division of a precursor cell, the meristemoid mother cell (MMC), which gives rise to two daughter cells. One retains its stem-cell-like properties to continue the stomatal lineage, while the other differentiates into a pavement cell or a guard mother cell (GMC) (Chen, L., 2023). The GMC undergoes a final symmetric division to produce two guard cells, which together frame a functional stoma (Nadeau & Sack, 2002).
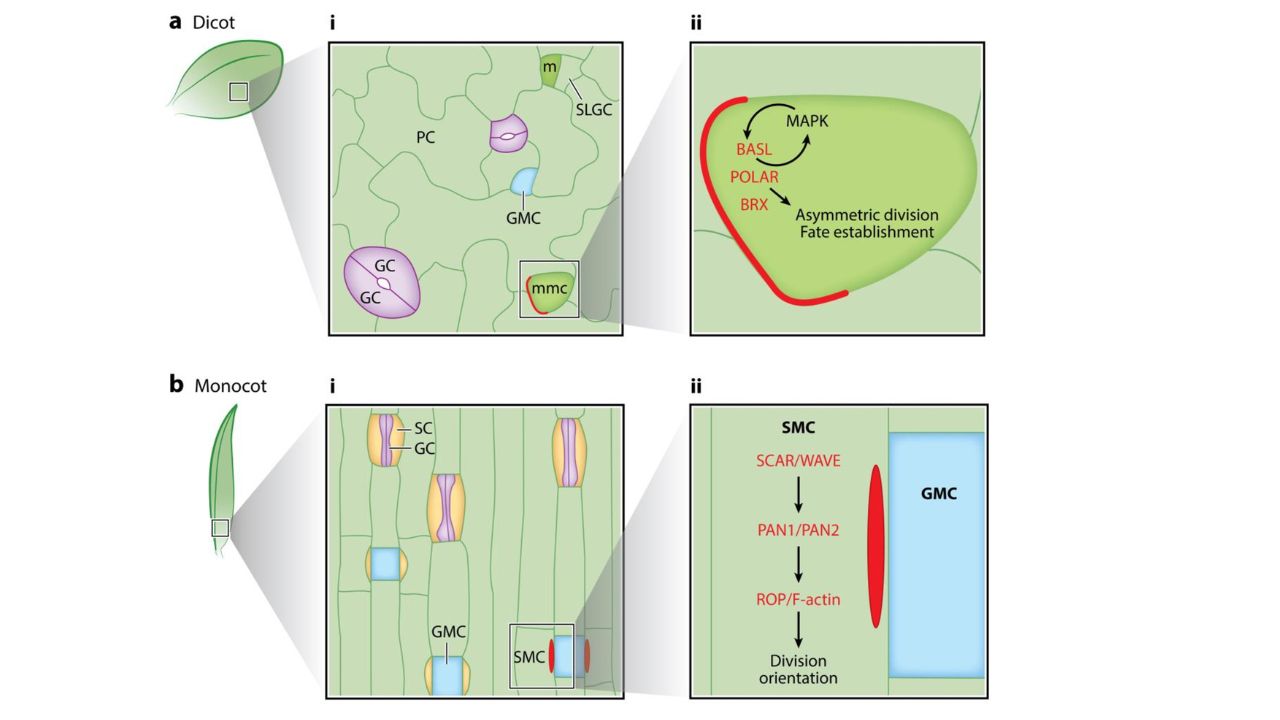
Fig.2. Cell polarity drives asymmetric cell division during stomatal formation in dicots and monocots. (a) In dicots, stomata are spaced throughout the leaf epidermis. (i) Stomatal meristemoid mother cells (mmc, boxed) generate stomatal lineage ground cells (SLGCs), which will differentiate into pavement cells (PCs), and meristemoids (m, bright green), which eventually transition into guard mother cells (GMCs, light blue). GMCs divide to generate the two guard cells (GCs, purple). (ii) In meristemoids, a polarity domain defined by BASL, BRX, and POLAR (red) drives asymmetric cell division and differential fate in daughter cells. Positive feedback between BASL and MAPK is critical for polarity establishment and fate establishment. (b) In monocots, stomata are arranged in cell files. (i) GCs are flanked by subsidiary cells (SCs, yellow). (ii) A ROP-dependent polarity cascade (red) in SMCs orients asymmetric divisions to generate SCs (Muroyama & Bergmann, 2019).
Proper spacing and differentiation of stomata are critical for their functionality and rely on tightly controlled patterning mechanisms. These mechanisms involve signaling pathways and molecular processes that coordinate cell fate determination, division orientation, and differentiation (Bergmann & Sack, 2007). Cellular polarity plays a pivotal role in ensuring that molecular cues and proteins are asymmetrically distributed within cells (Sampathkumar et al., 2014). This asymmetric localization drives the orientation of cell divisions and determines the fate of daughter cells, underscoring its importance in forming stomatal complexes (Sablowski & Gutierrez, 2022).
Recent studies highlight the role of protein kinases in establishing cellular polarity during stomatal development. For instance, the AGC kinase family, particularly D6PK, has been shown to rely on plasma membrane polarity mediated by PDK1-dependent phosphorylation (Graf et al., 2024). Furthermore, the POLAR guided signaling complex has been identified as a key regulator of asymmetric divisions within the stomatal lineage, coordinating protein localization and activity (Houbaert et al., 2018). Despite these advancements, the precise molecular mechanisms that integrate polarity signals with cell division and differentiation remain areas of active research.
Protein Kinases in Plant Development
Protein kinases function as molecular switches in diverse signaling cascades by phosphorylating specific target proteins to modulate their activity, localization, or stability (Stone & Walker, 1995; Wang, et al. 2007). In plants, these enzymes play pivotal roles in essential processes such as growth, development, stress responses, and cellular polarity (Hardie, 1999).
AGC (cAMP-dependent, cGMP-dependent, and protein kinase C-like) kinases represent a family of serine/threonine kinases that have gained attention for their involvement in polarity regulation and cell division (Zhang et al., 2019). Within this family, AGC1 kinases are specifically implicated in the spatial distribution of proteins and signaling components, ensuring cellular asymmetry (Graf et al., 2024).
In the context of stomatal development, AGC1 kinases interact with critical polarity proteins, such as BASL and POLAR, to influence asymmetric cell division (Houbaert et al., 2018). These interactions ensure that signaling complexes are correctly assembled and localized, enabling precise regulation of cell polarity (Zhang et al., 2019). Despite these insights, the exact molecular mechanisms by which AGC1 kinases coordinate these processes remain to be fully elucidated, making them a focal point of ongoing research.
Polarity Mechanisms in Plants
Polarity in plant cells is established and maintained by a network of proteins that localize asymmetrically within the cell. Among these, BASL (BREAKING OF ASYMMETRY IN THE STOMATAL LINEAGE) and POLAR are key regulators of polarity in the stomatal lineage (Dong et al., 2009; Zhang et al., 2019).
- BASL: This protein localizes asymmetrically during stomatal development and acts as a scaffold to recruit other signaling proteins, guiding the orientation of cell divisions (Dong et al., 2009). Its dynamic distribution within cells is critical for establishing polarity and ensuring proper stomatal patterning (Pillitteri & Torii, 2012; Houbaert et al., 2018).
- POLAR: Working in conjunction with BASL, POLAR stabilizes and enhances polarity. It interacts with protein kinases, such as AGC1 kinases, to regulate their localization and activity, further ensuring the fidelity of asymmetric cell divisions (Zhang et al., 2019; Graf et al., 2024).
The interplay between these polarity proteins and signaling pathways, including protein kinases, is central to stomatal development. AGC1 kinases are hypothesized to modulate these interactions by phosphorylating components of the polarity complex, thereby influencing their localization and function. These regulatory mechanisms highlight the intricate coordination required to maintain cellular asymmetry during development.
Research Gaps
While significant progress has been made in understanding the molecular mechanisms underlying stomatal development, key gaps remain:
Integration of AGC1 Kinases into Polarity Pathways
Although AGC1 kinases are known to play a role in polarity and cell division, how they integrate with known polarity proteins like BASL and POLAR during stomatal development is not well understood. The molecular interactions and downstream signaling pathways involving AGC1 kinases and these polarity regulators remain elusive.
Mechanistic Insights into AGC1 Function
The specific contributions of AGC1 kinases in asymmetric cell division and stomatal differentiation require further investigation. Detailed mechanistic studies are needed to uncover their substrates, interaction partners, and regulatory mechanisms during stomatal lineage development.
Hypothesis
AGC1 kinases regulate stomatal development by modulating the activity of key polarity proteins, thereby influencing asymmetric cell divisions and guard cell differentiation.
Objectives
- To characterize the expression and localization patterns of AGC1 kinases during stomatal development.
- To investigate the interaction of AGC1 kinases with known polarity proteins (e.g., BASL, POLAR).
- To analyze the impact of AGC1 kinase perturbation (via mutants or overexpression lines) on stomatal lineage cell polarity and division.
- To elucidate downstream signaling pathways mediated by AGC1 kinases.
Materials and Methods
Plant Material
Model Plant: Arabidopsis thaliana
Arabidopsis thaliana is selected as the model organism due to its well-annotated genome, ease of genetic manipulation, and established resources for studying stomatal development. Its short life cycle and small size make it ideal for experimental work in molecular biology.
Mutant and Transgenic Lines
Specific genetic lines will be used to dissect the role of AGC1 kinases in stomatal development:
- AGC1 kinase knockouts: Arabidopsis mutants lacking functional AGC1 kinase genes will be employed to investigate their phenotypic consequences on stomatal development and polarity.
- BASL-GFP and POLAR-mCherry reporters: These transgenic lines, expressing BASL and POLAR fused with fluorescent tags, will be utilized to monitor their localization and dynamics in vivo during stomatal lineage progression.
Molecular and Cellular Techniques
Gene Expression Analysis
Quantitative PCR (qPCR)
AGC1 kinase expression patterns will be quantified at different developmental stages and under varying environmental conditions. Tissue-specific expression will also be assessed.
In situ hybridization
Spatial expression of AGC1 kinases will be visualized in stomatal lineage cells, providing insights into their roles during specific developmental stages.
Protein Localization
Confocal Microscopy: AGC1 kinases tagged with fluorescent proteins (e.g., GFP or RFP) will be expressed in Arabidopsis. Confocal imaging will be used to determine their subcellular localization and dynamic changes during stomatal development. Co-localization with polarity proteins such as BASL and POLAR will also be analyzed.
Genetic Interaction Studies
Generation of Double Mutants
Double mutants (e.g., agc1 × basl) will be created through genetic crosses to study epistatic relationships. Phenotypic analysis of these mutants will help elucidate the genetic hierarchy between AGC1 kinases and known polarity regulators.
Complementation Assays
Kinase-dead AGC1 variants (non-functional mutants) will be introduced into AGC1 knockout backgrounds. Rescue of phenotypes will confirm the functional necessity of AGC1 kinase activity in stomatal development.
Functional Assays
Stomatal Density and Distribution Analysis
Leaves from wild-type, AGC1 knockout, and overexpression lines will be imaged and analyzed to quantify stomatal density and spatial distribution. Abnormal patterns will indicate disrupted polarity and division regulation.
Live-Cell Imaging
Time-lapse imaging of developing stomata will be performed to monitor cell division orientation, polarity establishment, and protein dynamics in real-time. Fluorescent reporters for AGC1 kinases, BASL, and POLAR will be used for this purpose.
Omics Approaches
Phosphoproteomics
Proteins from stomatal lineage cells will be extracted and analyzed using mass spectrometry to identify phosphorylation targets of AGC1 kinases. Enrichment of phosphorylation motifs specific to AGC1 activity will reveal potential downstream effectors.
Transcriptomics
- RNA sequencing will be conducted on stomatal lineage cells from wild-type and AGC1 mutant lines. Differential gene expression analysis will identify pathways and regulatory networks influenced by AGC1 kinase activity. Integration with phosphoproteomics data will provide a comprehensive view of AGC1-mediated signaling.
Work Plan
Year 1: Preliminary Studies and Establishment
- Generation of Research Materials
- Develop and validate transgenic Arabidopsis lines expressing fluorescently tagged AGC1 kinases (e.g., AGC1-GFP) and generate knockout mutants for AGC1 kinase genes.
- Cross existing BASL-GFP and POLAR-mCherry lines with AGC1 kinase mutants to enable co-localization and interaction studies.
- Expression and Localization Studies
- Perform quantitative PCR and in situ hybridization to characterize the spatiotemporal expression of AGC1 kinases in stomatal lineage cells.
- Use confocal microscopy to determine subcellular localization and dynamic behavior of AGC1 kinases during stomatal development.
Year 2: Functional and Interaction Analysis
- Genetic Interaction Studies
- Generate and analyze double mutants (e.g., agc1 × basl or agc1 × polar) to explore genetic interactions and their impact on stomatal patterning and development.
- Conduct complementation assays with kinase-dead AGC1 variants to confirm the role of kinase activity in stomatal development.
- Functional Characterization
- Quantify stomatal density, spacing, and division patterns in AGC1 knockout and overexpression lines.
- Perform live-cell imaging to study polarity dynamics and division orientation in real-time using fluorescently tagged AGC1, BASL, and POLAR reporters.
Year 3: Mechanistic Insights and Data Integration
- Omics Approaches
- Use phosphoproteomics to identify phosphorylation targets of AGC1 kinases and validate potential substrates involved in polarity regulation.
- Conduct transcriptomic analysis of stomatal lineage cells from wild-type and AGC1 mutants to uncover gene expression changes and regulatory pathways influenced by AGC1 kinases.
- Data Integration and Model Development
- Synthesize findings from genetic, molecular, and omics studies to construct a comprehensive working model of AGC1 kinase function in regulating polarity and stomatal development.
- Dissemination of Results
- Begin drafting manuscripts for publication in peer-reviewed journals.
- Present findings at relevant conferences and symposia.
References
Bergmann, D. C., and Sack, F. D. (2007). Stomatal development. Annual Review of Plant Biology, 58, 163-181.
Chen, L. (2023). Emerging roles of protein phosphorylation in regulation of stomatal development. Journal of Plant Physiology, 280.
Dong J, MacAlister CA, Bergmann DC (2009) BASL controls asymmetric cell division in Arabidopsis. Cell 137: 1320–1330.
Graf, A., Bassukas, A.E.L., Xiao, Y., Barbosa, I.C.R., Mergner, J., Grill, P., Michalke, B., Kuster, B., and Schwechheimer, C. (2024). D6PK plasma membrane polarity requires a repeated CXX(X)P motif and PDK1-dependent phosphorylation. Nat Plants 10, 300-314.
Hammes, U.Z., Murphy, A.S., and Schwechheimer, C. (2022). Auxin Transporters – A Biochemical View. Cold Spring Harb Perspect Biol 14.
Hardie, D. (1999). Plant Protein Serine/ Threonine Kinases: Classification and Functions. Annual Review of Plant Biology 50, 97 – 131.
Houbaert, et al., (2018). POLAR guided signaling complex assembly and localization drive asymmetric cell division. Nature, 563, 574-578.
Janacek, D.P., Kolb, M., Schulz, L., Mergner, J., Kuster, B., Glanc, M., Friml, J., Ten Tusscher, K., Schwechheimer, C., and Hammes, U.Z. (2024). Transport properties of canonical PIN-FORMED proteins from Arabidopsis and the role of the loop domain in auxin transport. Dev Cell S1534-5807(24)00569-0.
Koh, S.W.H., Marhava, P., Rana, S., Graf, A., Moret, B., Bassukas, A.E.L., Zourelidou, M., Kolb, M., Hammes, U.Z., Schwechheimer, C., and Hardtke, C.S. (2021). Mapping and engineering of auxin-induced plasma membrane dissociation in BRX family proteins. Plant Cell 33, 1945-1960.
Marhava, P., Bassukas, A.E.L., Zourelidou, M., Kolb, M., Moret, B., Fastner, A., Schulze, W.X., Cattaneo, P., Hammes, U.Z., Schwechheimer, C., and Hardtke, C.S. (2018). A molecular rheostat adjusts auxin flux to promote root protophloem differentiation. Nature 558, 297-300.Muroyama, A., and Bergmann D. (2019). Plant Cell Polarity: Creating Diversity from Inside the Box. Annual Review, 35, 309-336.
Nadeau, J. A., and Sack, D.F., (2002). “Stomatal development in Arabidopsis.” The Arabidopsis book/American Society of Plant Biologists 1.
Pillitteri, L., and Torii, K. (2012). Mechanisms of Stomatal Development. Annual Review of Plant Biology 63:591-614.
Sablowski, R., and Gutierrez, C. (2022). Cycling in a crowd: Coordination of plant cell division, growth, and cell fate. The Plant Cell, 34 (1), 193–208.
Sampathkumar, A., Yan, A., & Krupinski, P. (2014). Physical Forces Regulate Plant Development and Morphogenesis. Current Biology, 24(10), 475-483.
Stone, J.M., and Walker, J.C. (1995). Plant Protein Kinase Families and Signal Transduction. Plant Physiol. 108, 451- 457.
Wang et al. (2007). “The protein phosphatases and protein kinases of Arabidopsis thaliana.” The Arabidopsis Book/American Society of Plant Biologists 5 (2007).
Xiao, Y., Zourelidou, M., Bassukas, A.E.L., Weller, B., Janacek, D.P., Schulz, L., Brajkovic, S., Šimura, J., Ljung, K., Kuster, B., Hammes, U.Z., Li, J., and Schwechheimer, C. (2024). KIPK and KIPK-LIKE1 suppress overbending during negative hypocotyl gravitropic growth. bioRxiv 2024.2005.2024.595653
Zhang, Z., Zhong, Z., and Xiong, Y. (2023). Sailing in complex nutrient signaling networks: Where I am, where to go, and how to go? Molecular Plant,16(10), 1635-1660.



Pingback: Phospho-Regulation of the Plant Cytoskeleton: Analysis of Plant Growth Processes Regulated by AGC1 Kinases. -
Pingback: Developmental Adaptations for Plant Growth in Soil: Regulated by AGC1 Kinases -