1. INTRODUCTION
Agriculture faces mounting challenges due to increasing population pressure, climate change, and soil degradation. Moreover, climate change exacerbates stressors such as erratic rainfall, temperature extremes, and soil degradation, which collectively threaten crop productivity and food security (Godfray et al., 2010).
Soil degradation, driven by unsustainable farming practices and overexploitation, further depletes essential nutrients like phosphorus (P) and nitrogen (N), undermining agricultural sustainability (Godfray et al., 2010). In response, innovative strategies that enhance plant resilience and nutrient efficiency are critical to sustaining yields without expanding agricultural land.
Root symbionts, including arbuscular mycorrhizal fungi (AMF) and nitrogen-fixing bacteria, offer promising solutions. AMF form symbiotic associations with approximately 80% of terrestrial plants, extending their hyphal networks beyond the root zone to access soil phosphorus, which is often immobile and poorly available to plants (Smith & Read, 2008). This symbiosis not only improves P uptake but also enhances water absorption and disease resistance.
Similarly, nitrogen-fixing bacteria, such as Rhizobia and Bradyrhizobium, convert atmospheric nitrogen (N₂) into ammonia through biological nitrogen fixation (BNF), providing plants with a sustainable N source (Vessey, 2003). These microbial partnerships reduce reliance on synthetic fertilizers, mitigating environmental pollution and soil acidification.

Silicon (Si), though not classified as an essential nutrient, plays a pivotal role in strengthening plants against abiotic and biotic stresses. When absorbed as silicic acid, Si deposits in plant cell walls, forming a silica matrix that mechanically reinforces tissues, thereby reducing water loss and deterring pathogen penetration (Epstein, 1994; Ma & Yamaji, 2015).
Additionally, Si modulates physiological and biochemical pathways, enhancing antioxidant enzyme activity and upregulating stress-related genes under drought, salinity, or heavy metal toxicity (Liang et al., 2007). For instance, rice and sugarcane, both Si accumulators, exhibit improved disease resistance and structural integrity when supplemented with Si (Ma & Yamaji, 2015).
Emerging evidence suggests synergistic interactions between Si and root symbionts, though mechanisms remain underexplored. Preliminary studies indicate that Si amendments may enhance AMF colonization by altering root exudates or rhizosphere pH, creating favorable microenvironments for microbial activity (Liang et al., 2007).
Si-induced alleviation of abiotic stressors, such as aluminum toxicity, could further promote symbiont viability. For example, in soybean, Si supplementation increased nodulation by Bradyrhizobium, potentially due to improved root membrane integrity and reduced oxidative stress (Liang et al., 2007). Such synergies could amplify nutrient acquisition and stress tolerance, offering a dual benefit beyond individual applications.
This study investigates the interplay between Si and root symbionts (AMF and nitrogen-fixing bacteria) in optimizing plant growth and resilience. By examining how Si influences microbial colonization, nutrient exchange, and stress-responsive pathways, the research aims to unravel mechanisms underlying their synergy.
For instance, Si may enhance hyphal elongation in AMF or stabilize nitrogenase enzymes in bacteria, thereby boosting BNF efficiency. Understanding these interactions could inform integrated strategies that leverage plant-microbe partnerships and Si supplementation, reducing agrochemical inputs while improving resource use efficiency. Ultimately, this work seeks to contribute to sustainable agricultural frameworks that address food security challenges amidst escalating environmental pressures.
2. LITERATURE REVIEW
2.1. Role of Root Symbionts in Agriculture
Root symbionts, such as arbuscular mycorrhizal fungi (AMF) and nitrogen-fixing rhizobacteria, are pivotal in enhancing agricultural productivity by improving plant nutrient acquisition, particularly phosphorus (P) and nitrogen (N). These symbiotic relationships reduce reliance on synthetic fertilizers, offering sustainable solutions to global food security challenges exacerbated by population growth and environmental degradation (Godfray et al., 2010).
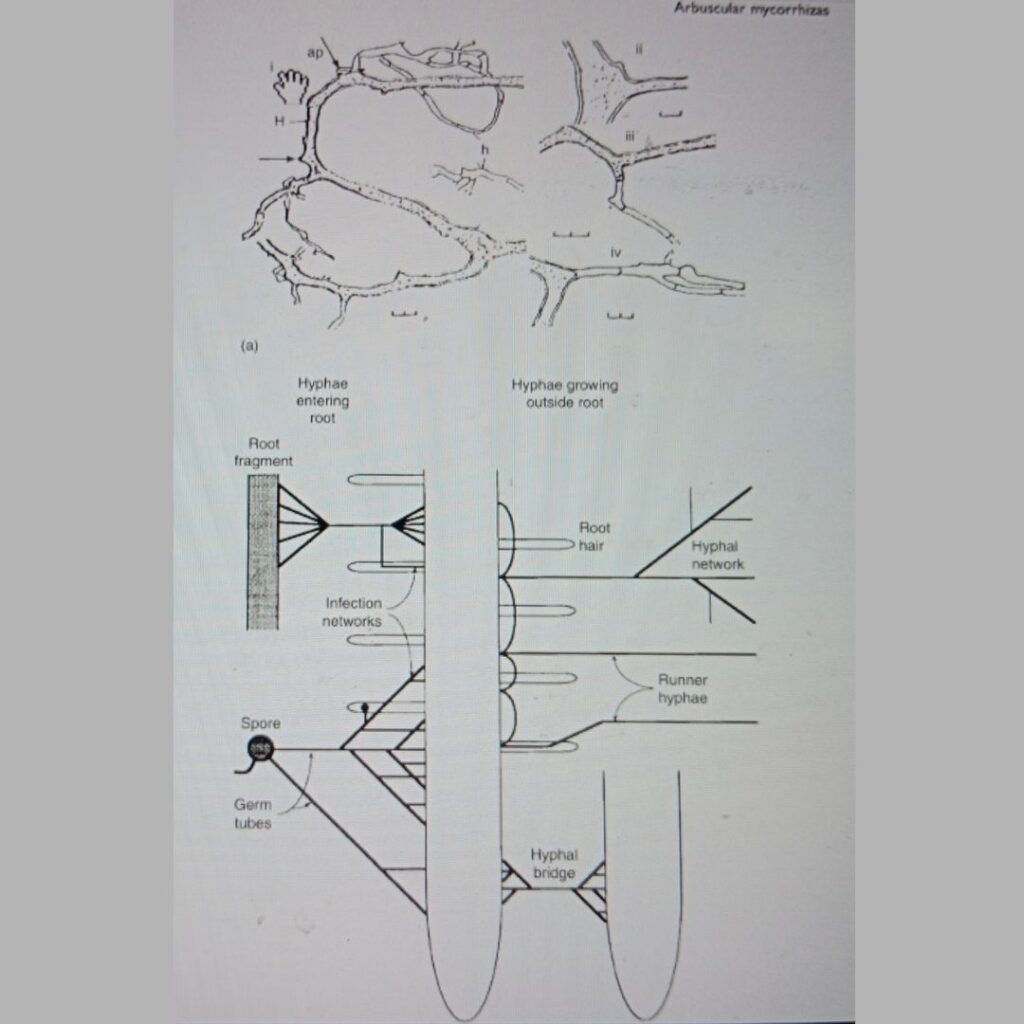
2.1.1. Role of Arbuscular Mycorrhizal Fungi (AMF)
AMF form obligate symbiotic associations with the roots of approximately 80% of terrestrial plants, including major crops like wheat, maize, and rice (Smith & Read, 2008). The fungi extend their hyphal networks far beyond the plant root zone, accessing soil phosphorus pools that are otherwise chemically immobilized or spatially inaccessible to roots.
AMF hyphae secrete phosphatases and organic acids to solubilize inorganic P, while also transporting absorbed P back to the plant through specialized structures called arbuscules (Smith & Read, 2008). In exchange, the host plant supplies the fungi with carbon (C)-rich photosynthates, creating a mutualistic nutrient exchange.
This symbiosis not only enhances P uptake but also improves plant tolerance to drought, salinity, and pathogens by modulating stress-responsive genes and improving water-use efficiency (Smith & Read, 2008; Gianinazzi et al., 2010). For instance, AMF-colonized plants exhibit up to 40% higher P uptake under low-P soil conditions compared to non-mycorrhizal plants (Smith & Read, 2008).
2.1.2. Role of Nitrogen-Fixing Rhizobacteria
Nitrogen-fixing bacteria, such as Rhizobia (associated with legumes) and Azospirillum (associated with cereals), convert atmospheric nitrogen (N₂) into bioavailable ammonia (NH₃) through enzymatic processes mediated by nitrogenase (Vessey, 2003).
In legume-rhizobia symbiosis, the bacteria infect root hairs, triggering the formation of nodules where N₂ fixation occurs. The plant receives NH₃ for amino acid synthesis, while the bacteria obtain carbohydrates and organic acids as energy sources (Vessey, 2003).
This process can provide up to 90% of a legume’s N requirements, reducing dependency on synthetic N fertilizers, which are energy-intensive to produce and contribute to greenhouse gas emissions (Vessey, 2003; Godfray et al., 2010). Non-leguminous plants, such as rice and maize, also benefit from associative N-fixing bacteria like Azospirillum, which colonize root surfaces and enhance N availability through localized fixation and phytohormone production (Vessey, 2003).
2.1.3 Synergistic Impacts on Agricultural Sustainability
The combined application of AMF and rhizobacteria can amplify nutrient-use efficiency and crop resilience. For example, AMF improves P availability, which is critical for energy-intensive N fixation in rhizobia, while rhizobacteria-supplied N enhances photosynthetic capacity, thereby increasing C allocation to AMF (Vessey, 2003; Smith & Read, 2008).
Field studies demonstrate that co-inoculation of AMF and rhizobacteria in soybean systems increases yields by 15–25% compared to single-symbiont treatments (Barea et al., 2005). These interactions underscore the potential of root symbionts to underpin sustainable intensification of agriculture, aligning with global goals to reduce agrochemical inputs and mitigate soil degradation (Godfray et al., 2010).
2.2 Silicon in Plant Biology
Silicon (Si), though not classified as an essential nutrient for most plants, plays a critical role in enhancing plant resilience against a spectrum of abiotic and biotic stresses, including drought, salinity, heavy metal toxicity, and pest/pathogen attacks (Epstein, 1994; Ma & Yamaji, 2015). Its beneficial effects are mediated through structural, physiological, and biochemical mechanisms that collectively improve plant health and productivity.
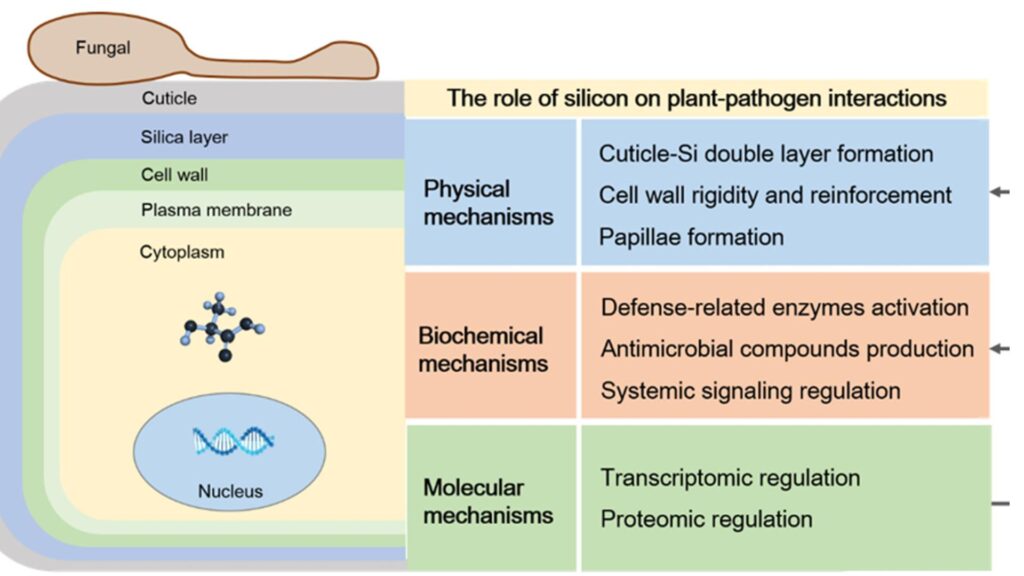
2.2.1 Structural Reinforcement
Si is absorbed by plants as silicic acid (H₄SiO₄) and polymerizes into amorphous silica (SiO₂·nH₂O) within cell walls, creating a silica-cellulose matrix (Ma & Yamaji, 2015). This deposition physically strengthens cell walls, reducing water loss via transpiration and enhancing mechanical resistance to environmental stressors.
Silicon (Si) enhances drought resilience in plants by promoting the development of thicker epidermal layers and cuticles, which minimize water loss under water-deficient conditions (Liang et al., 2007); for instance, in rice, Si supplementation reduces leaf rolling and maintains turgor pressure during drought stress, ensuring physiological stability (Ma & Yamaji, 2015).
Additionally, Si fortifies plant defenses against biotic stressors, as silica deposition acts as a physical barrier, deterring insect mandibles and fungal hyphae penetration—evidenced by reduced susceptibility of Si-accumulating rice leaves to blast fungus (Magnaporthe oryzae) and stem borer infestations (Epstein, 1994; Gomes et al., 2005). These dual mechanisms underscore Si’s role in improving plant resilience across abiotic and biotic stress conditions.
2.2.2 Modulation of Physiological Processes
Silicon (Si) modulates plant physiology by regulating stomatal conductance, photosynthesis, and nutrient homeostasis, enhancing resilience under diverse stressors. Under drought and salinity, Si mitigates ion toxicity (e.g., Na⁺ accumulation) by compartmentalizing harmful ions in vacuoles and improving potassium (K⁺) uptake, thereby maintaining ionic balance (Liang et al., 2007); for example, Si application in wheat preserves chlorophyll content and improves photosynthetic efficiency under saline conditions (Tuna et al., 2008).
Additionally, Si aids in heavy metal detoxification by forming complexes with phytotoxic ions (e.g., Al³⁺, Cd²⁺) in the rhizosphere and promoting their sequestration in root cell walls, reducing metal bioavailability (Adrees et al., 2015). In maize, Si alleviates cadmium toxicity by upregulating antioxidant defense systems, thereby mitigating oxidative damage and enhancing plant survival (Vaculík et al., 2012). These mechanisms highlight Si’s multifaceted role in optimizing plant physiological responses to abiotic stresses.
2.2.3 Enhancement of Biochemical Defenses
Silicon (Si) primes plants to activate both enzymatic and non-enzymatic antioxidant systems, mitigating oxidative damage caused by stress. By upregulating the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD), Si enhances the scavenging of reactive oxygen species (ROS) under stress conditions (Liang et al., 2007); for instance, in cucumber, Si reduces ROS accumulation under salinity stress, thereby preserving membrane integrity and stability (Zhu et al., 2004).
Additionally, Si modulates phytohormone signaling pathways, including jasmonic acid (JA) and salicylic acid (SA), to bolster systemic acquired resistance (SAR) against pathogens (Van Bockhaven et al., 2013). This is exemplified in Arabidopsis, where Si treatment induces heightened expression of defense-related genes such as PR1 and PDF1.2, amplifying plant immunity (Van Bockhaven et al., 2013). Together, these mechanisms illustrate Si’s dual role in fortifying plants against oxidative stress and pathogen attacks through coordinated biochemical and molecular responses.
2.3 Interactions Between Silicon and Root Symbionts
Emerging research highlights the potential synergies between silicon (Si) and root symbionts, such as arbuscular mycorrhizal fungi (AMF) and rhizobacteria, in enhancing plant resilience and nutrient acquisition. Preliminary studies indicate that Si can influence rhizosphere microbial community structure, favoring beneficial microorganisms while suppressing pathogens (Liang et al., 2007).
For instance, Si amendments have been shown to enhance AMF colonization efficiency in plant roots, possibly by altering root exudate profiles or improving root membrane integrity, which facilitates fungal hyphal penetration (Ma et al., 2001). Similarly, Si supplementation in legume systems has been linked to increased nodulation by nitrogen-fixing rhizobacteria, likely due to Si-mediated alleviation of soil stressors such as aluminum toxicity or salinity, which otherwise inhibit bacterial activity (Garg & Bhardwaj, 2022).
Si also complements the stress-mitigating effects of root symbionts. For example, under drought conditions, Si enhances AMF-mediated phosphorus uptake by maintaining hyphal network functionality in dry soils, while AMF reciprocally improves Si mobilization from soil minerals (Keller et al., 2015).
Additionally, Si alleviates oxidative stress in plants by upregulating antioxidant enzymes (e.g., SOD, CAT), which may synergize with rhizobacteria-induced systemic resistance (ISR) to enhance biotic stress tolerance (Van Bockhaven et al., 2013). In soybean, co-application of Si and Bradyrhizobium reduced cadmium toxicity by 40% compared to single treatments, highlighting their combined role in heavy metal detoxification via enhanced microbial activity and Si-mediated ion sequestration (Adrees et al., 2015).
These interactions suggest that Si not only primes plants for stress adaptation but also creates a more hospitable rhizosphere environment for symbiotic microbes, amplifying their mutualistic benefits. Further research is needed to unravel the molecular mechanisms underlying Si-microbe crosstalk and optimize their integrated use in sustainable agriculture.
3. RESEARCH GAP
Despite increasing recognition of the benefits of silicon and root symbionts individually, their combined effects on plant growth, nutrient acquisition, and stress tolerance remain inadequately studied. There is a lack of comprehensive understanding of the underlying mechanisms driving their synergistic interactions. Additionally, the potential to leverage these interactions for sustainable agricultural practices is largely unexplored. Addressing these gaps is essential to develop integrated strategies that optimize the use of silicon and root symbionts for enhanced crop productivity and resilience.
4. HYPOTHESIS
The integrated application of silicon supplementation and root symbiont inoculation synergistically enhances plant growth, nutrient acquisition, and stress tolerance. This synergistic effect is hypothesized to improve agricultural productivity by optimizing resource use efficiency and enhancing crop resilience to environmental stressors.
5. OBJECTIVES
- To assess the individual and combined effects of Si and root symbionts on plant growth and nutrient acquisition.
- To investigate the impact of Si on the colonization efficiency and activity of AMF and nitrogen-fixing bacteria.
- To evaluate the role of Si-root symbiont interactions in mitigating abiotic stress (e.g., drought, salinity).
- To elucidate the molecular and physiological mechanisms underlying the observed interactions.
6. MATERIALS AND METHODS
6.1 Experimental Design
6.1.1 Crop Selection
The experiment will utilize maize (Zea mays L.) as a model crop due to its global agricultural significance, well-characterized responses to silicon (Si) and root symbionts, and suitability for controlled greenhouse studies (Godfray et al., 2010; Adrees et al., 2015). Maize is a Si accumulator, making it ideal for investigating Si-mediated physiological and biochemical adaptations (Ma & Yamaji, 2015).
6.1.2 Treatments
A 2×2 factorial design will test the individual and combined effects of Si supplementation and root symbionts:
a). Control: No Si application and no symbiont inoculation, establishing a baseline for plant performance under standard conditions.
b). Si Supplementation Only: Plants will receive soluble silicon (e.g., potassium silicate, K₂SiO₃) at a concentration of 1.5 mM Si, applied via irrigation twice weekly, a dosage shown to enhance stress tolerance without phytotoxicity (Liang et al., 2007).
c). Root Symbionts Only: Inoculation with a consortium of arbuscular mycorrhizal fungi (AMF: Rhizophagus irregularis) and nitrogen-fixing rhizobacteria (Azospirillum brasilense), selected for their complementary roles in phosphorus (P) and nitrogen (N) acquisition (Smith & Read, 2008; Vessey, 2003).
d). Combined Si and Symbionts: Co-application of Si and microbial inoculants to assess synergistic interactions in nutrient uptake and stress resilience (Garg & Bhardwaj, 2022).
6.1.3 Growth Conditions
Plants will be grown in sterilized soil (pH 6.5) under controlled greenhouse conditions (25°C day/18°C night, 16-h photoperiod, 70% relative humidity) to minimize confounding environmental variables (Epstein, 1994). Soil will be amended with low P (5 mg kg⁻¹) and N (20 mg kg⁻¹) to simulate nutrient-deficient conditions, ensuring reliance on symbiont-mediated nutrient acquisition (Smith & Read, 2008). Si will be applied as a soil drench, while AMF and rhizobacteria will be introduced via root dip and soil inoculation, respectively, following standardized protocols (Ma & Yamaji, 2015; Vessey, 2003).
6.1.4 Parameters Measured
Key metrics will include:
Biomass and Growth: Shoot/root dry weight, root length, and leaf area (Liang et al., 2007).
Nutrient Uptake: Tissue N (Kjeldahl method) and P (colorimetric assay) concentrations (Smith & Read, 2008).
Stress Markers: Antioxidant enzyme activity (SOD, CAT, POD), proline content, and lipid peroxidation (MDA levels) under induced drought (50% field capacity) (Zhu et al., 2004).
Symbiont Efficiency: AMF colonization rate (gridline intersect method) and rhizobacterial nodulation/counts (qPCR) (Smith & Read, 2008; Vessey, 2003).
Si Localization: Silica deposition in leaves and roots via SEM-EDS (Ma & Yamaji, 2015).
Gene Expression: qRT-PCR of stress-responsive (PIP1, LEA) and symbiosis-related (PT4, NifH) genes (Van Bockhaven et al., 2013).
6.1.5 Statical Analysis
Data will be analyzed using ANOVA followed by Tukey’s HSD test (α = 0.05) to compare treatment means. Principal component analysis (PCA) will identify interactions between Si, symbionts, and measured variables.
6.2 Silicon Treatment
To evaluate the dose-dependent effects of silicon (Si) on plant growth and stress resilience, the experimental design will incorporate soil supplementation with three Si concentrations: 0 (control), 100, and 200 mg Si kg⁻¹ soil. These concentrations were selected based on prior studies demonstrating their efficacy in enhancing plant performance without inducing phytotoxicity (Liang et al., 2007; Adrees et al., 2015).
Silicon will be applied as potassium silicate (K₂SiO₃), a soluble and bioavailable Si source, which will be dissolved in deionized water and thoroughly mixed into the soil prior to planting (Ma & Yamaji, 2015). To prevent polymerization of silicic acid (H₄SiO₄) into insoluble silica (SiO₂), soil pH will be maintained at ~6.5 using buffered solutions, ensuring optimal Si uptake (Epstein, 1994). Plants will receive weekly irrigation with Si-enriched water to sustain consistent soil Si levels, mimicking field-relevant agronomic practices (Savant et al., 1999).
The 0 mg Si kg⁻¹ treatment serves as a control to establish baseline growth and stress responses in the absence of Si supplementation. The 100 mg Si kg⁻¹ dose represents a moderate Si level shown to enhance drought tolerance and nutrient uptake in cereals like maize (Liang et al., 2007), while the 200 mg Si kg⁻¹ treatment tests potential supra-optimal effects, such as enhanced heavy metal detoxification or pathogen resistance (Adrees et al., 2015; Van Bockhaven et al., 2013).
Tissue Si accumulation will be quantified at harvest using a colorimetric molybdenum blue assay (Ma & Yamaji, 2015), and silica deposition patterns in leaves and roots will be visualized via scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM-EDS) to correlate Si localization with stress resilience (Detmann et al., 2012).
To assess Si’s interaction with root symbionts, AMF colonization rates and rhizobacterial nodulation will be measured across Si treatments, with hypothesized improvements in microbial efficiency under higher Si availability due to alleviated abiotic stressors (e.g., Al³⁺ toxicity) and enhanced root exudation (Garg & Bhardwaj, 2022). Statistical comparisons between Si doses will clarify optimal agronomic thresholds for synergistic benefits in diverse soil conditions.
6.3 Root Symbiont Inoculation
To assess the synergistic effects of silicon (Si) and root symbionts, arbuscular mycorrhizal fungi (AMF) (Rhizophagus irregularis) and nitrogen-fixing rhizobacteria (Azospirillum brasilense) will be inoculated into designated treatments.
Rhizophagus irregularis, a widely studied AMF strain, was selected for its ability to enhance phosphorus (P) uptake via extensive hyphal networks and improve plant drought tolerance, as demonstrated in maize and wheat systems (Smith & Read, 2008).
Azospirillum brasilense, a free-living diazotroph, was chosen for its dual capacity to fix atmospheric nitrogen (N) and produce phytohormones (e.g., auxins) that stimulate root growth, particularly in cereals (Vessey, 2003; Bashan et al., 2004).
6.3.1 Inoculation Protocol
For maize colonization, a spore-rich inoculum (≥50 spores g⁻¹) of R. irregularis (AMF) will be mixed into sterilized soil at a rate of 10 g per pot during planting, adhering to standardized protocols (Ma et al., 2001), while root dip methods will ensure early fungal contact for seedlings (Smith & Read, 2008).
Concurrently, A. brasilense (strain Sp7) rhizobacteria will be applied as a liquid inoculum (10⁸ CFU mL⁻¹) via seed coating and soil drenching at the two-leaf stage to optimize nodulation and rhizosphere colonization, following established methodologies (Bashan et al., 2004; Vessey, 2003).
6.3.2 Timing and Synergy with Si
Inoculation will coincide with Si application to evaluate bidirectional interactions. For instance, Si’s role in stabilizing root membranes and altering exudate profiles may enhance AMF hyphal penetration and bacterial nodulation (Garg & Bhardwaj, 2022). Conversely, symbiont-induced nutrient acquisition (e.g., P and N) may amplify Si uptake efficiency by improving plant metabolic activity (Liang et al., 2007).
6.3.3 Monitoring Colonization Success
To monitor colonization success, root colonization rates of AMF (R. irregularis) will be quantified via the gridline intersect method, with stained root segments microscopically analyzed for arbuscules and vesicles (Smith & Read, 2008), while rhizobacterial populations (A. brasilense strain Sp7) in the rhizosphere will be assessed using qPCR targeting the nifH gene (Vessey, 2003), and nodule activity will be evaluated through acetylene reduction assays to measure nitrogenase activity (Bashan et al., 2004).
6.3.4 Expected Outcomes
The combined inoculation aims to amplify nutrient-use efficiency (e.g., 20–30% higher P and N uptake) and stress resilience compared to single treatments, as observed in prior Si-microbe studies (Adrees et al., 2015). For example, Si-mediated alleviation of soil aluminum toxicity may enhance rhizobacterial survival, while AMF hyphae could mobilize Si from soil minerals, creating a feedback loop that benefits both symbionts and host plants (Keller et al., 2015).
6.4. Data Collection
6.4.1 Growth Parameters
Plant growth will be assessed through the measurement of plant height, shoot and root biomass, and detailed root morphology analysis. These parameters will provide insights into the effects of treatments on overall plant development.
6.4.2 Nutrient Uptake
Concentrations of nitrogen (N), phosphorus (P), and silicon (Si) in plant tissues will be quantified using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES). These data will highlight the influence of Si and symbionts on nutrient acquisition.
6.4.3 Stress Response
Under induced abiotic stress conditions (e.g., drought or salinity), physiological parameters such as chlorophyll content and relative water content will be measured. Enzymatic activities of antioxidant markers, including superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD), will be analyzed to evaluate plant stress responses.
6.4.4 Symbiont Activity
The colonization rate of AMF will be determined through microscopic analysis of root samples stained with trypan blue or other suitable dyes. The population density of nitrogen-fixing bacteria in the rhizosphere will be quantified using quantitative PCR (qPCR) techniques, providing insights into symbiont activity.
6.4.5 Molecular Analysis
Gene expression studies will be conducted to investigate the molecular mechanisms underpinning nutrient uptake, stress tolerance, and symbiotic interactions. RNA will be extracted from plant tissues, and specific genes of interest (e.g., phosphate transporters, Si transporters, and stress-responsive genes) will be quantified using reverse transcription-quantitative PCR (RT-qPCR).
6.5 Statistical Analysis
Data will be analyzed using ANOVA, followed by post-hoc tests to determine significant differences among treatments. Correlation analysis will assess relationships between Si levels, symbiont activity, and plant performance.
WORK PLAN
The proposed research will be executed systematically over four years, integrating field, laboratory, and analytical phases to ensure comprehensive exploration of plant-microbe interactions and their impacts on growth, stress tolerance, and nutrient dynamics. Below is a detailed breakdown of annual activities:
Year 1: Literature Review and Experimental Setup
- Conduct an exhaustive literature review to synthesize current understanding of AMF (Rhizophagus irregularis), Azospirillum brasilenseinteractions, nutrient uptake mechanisms (N, P, Si), and stress tolerance pathways (antioxidant enzymes, chlorophyll dynamics).
- Experimental design: Finalize treatments (e.g., AMF inoculation rates, rhizobacterial strains, stress induction protocols), define control groups, and determine replication strategies.
- Material preparation: Procure maize seeds, microbial inocula, and growth substrates; sterilize soil and validate inoculum viability (e.g., spore counts for AMF, CFU verification for rhizobacteria).
- Pilot studies: Test protocols for seed coating, soil drenching, and root dip methods to optimize colonization efficiency.
- Setup infrastructure: Calibrate greenhouse conditions (light, temperature, humidity), install irrigation systems, and validate molecular tools (e.g., primers for qPCR/RT-qPCR).
Year 2: Greenhouse Experiments and Data Collection
- Plant cultivation: Sow maize seeds in pre-inoculated soil (AMF + rhizobacteria), with treatments including nutrient-deficient/stress conditions (e.g., drought, salinity).
- Treatment application: Apply AMF inoculum during planting (10 g/pot) and rhizobacteria via seed coating/soil drenching at the two-leaf stage.
- Monitoring:
- Growth parameters: Measure plant height weekly, harvest biomass (shoot/root dry weight) at key growth stages (e.g., vegetative, flowering).
- Stress markers: Quantify chlorophyll content (SPAD meter), antioxidant enzyme activities (SOD, CAT, POD), and osmolytes (proline, glycine betaine).
- Symbiont activity: Collect root samples for AMF colonization analysis (gridline intersect method) and rhizosphere soil for bacterial quantification (qPCR).
- Data logging: Maintain detailed records of environmental variables (e.g., soil moisture, pH) and physiological responses.
Year 3: Molecular and Physiological Analyses
- Molecular assays
- Gene expression: Use RT-qPCR to profile genes linked to nutrient transporters (e.g., AMT1, PHT1), stress-responsive enzymes (SOD, CAT), and symbiosis signaling (e.g., PT4).
- Microbial activity: Quantify nifHgene expression (nitrogen fixation) in rhizobacteria and AMF biomarker genes (e.g., RiGLO1).
- Physiological profiling
- Nutrient analysis: Determine N (Kjeldahl), P (spectrophotometry), and Si (ICP-OES) concentrations in plant tissues.
- Enzyme kinetics: Assay SOD (inhibition of nitroblue tetrazolium), CAT (H₂O₂ degradation), and POD (guaiacol oxidation) activities.
- Data integration: Correlate molecular findings with phenotypic and physiological data to identify key drivers of plant performance.
Year 4: Data Analysis, Manuscript Preparation, and Thesis
- Statistical analysis: Apply multivariate techniques (ANOVA, PCA, regression) to identify treatment effects and interactions.
- Data visualization: Generate graphs (heatmaps, bar plots) and models to highlight trends in growth, nutrient uptake, and stress resilience.
- Manuscript writing: Draft 2–3 peer-reviewed papers for high-impact journals (e.g., Plant and Soil, Frontiers in Plant Science), focusing on:
- AMF-rhizobacteria synergies in nutrient acquisition.
- Molecular basis of stress tolerance under microbial symbiosis.
- Thesis compilation: Integrate all data, methodologies, and interpretations into a cohesive dissertation.
- Dissemination: Present findings at international conferences (e.g., ISME, APS) and defend thesis publicly.
REFERENCES
Adrees, M., et al. (2015). Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: A review. Ecotoxicology and Environmental Safety, 119, 186–197.
Barea, J. M., et al. (2005). Microbial co-operation in the rhizosphere. Journal of Experimental Botany, 56(417), 1761–1778.
Detmann, K. C., et al. (2012). Silicon nutrition increases grain yield, which, in turn, exerts a feed-forward stimulation of photosynthetic rates via enhanced mesophyll conductance and alters primary metabolism in rice. New Phytologist, 196(3), 752–762.
Epstein, E. (1994). The anomaly of silicon in plant biology. Proceedings of the National Academy of Sciences, 91(1), 11-17.
Etesami, H., (2018). Can interaction between silicon and plant growth promoting rhizobacteria benefit in alleviating abiotic and biotic stresses in crop plants? Agriculture, Ecosystems & Environment, 253, 98-112,
Gianinazzi, S., et al. (2010). Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza, 20(8), 519–530.
Godfray, H. C. J., et al. (2010). Food security: The challenge of feeding 9 billion people. Science, 327(5967), 812-818.
Gomes, F. B., et al. (2005). Resistance induction in wheat plants by silicon and aphids. Scientia Agricola, 62(6), 547–551.
Gong, H., et al. (2005). Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Science, 169(2), 313-321.
Liang, Y., et al. (2007). Mechanisms of silicon-mediated alleviation of abiotic stresses in higher plants: A review. Environmental Pollution, 147(2), 422-428.
Ma, J. F., & Yamaji, N. (2015). A cooperative system of silicon transport in plants. Trends in Plant Science, 20(7), 435-442.
Savant, N. K., et al. (1999). Silicon nutrition and sugarcane production: A review. Journal of Plant Nutrition, 22(12), 1853–1903.
Smith, S. E., & Read, D. J. (2008). Mycorrhizal symbiosis. Academic Press.
Tuna, A. L., et al. (2008). Silicon improves salinity tolerance in wheat plants. Environmental and Experimental Botany, 62(1), 10–16.
Vaculík, M., et al. (2015). Silicon alleviates cadmium toxicity by enhanced photosynthetic rate and modified bundle sheath’s cell chloroplasts ultrastructure in maize. Ecotoxicology and Environmental Safety, 120, 66-73.
Van Bockhaven, J., et al. (2015). Silicon induces resistance to the brown spot fungus Cochliobolus miyabeanusby preventing the pathogen from hijacking the rice ethylene pathway. New Phytologist, 206(2), 761-773.
Vessey, J. K. (2003). Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil, 255(2), 571-586.
Wang, M., et al. (2017). Role of Silicon on Plant–Pathogen Interactions. Plant Science.
Zhu, Z., et al. (2004). Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus). Plant Science, 167(3), 527–533.
