INTRODUCTION
Auxin and brassinosteroids (BRs) are pivotal plant hormones that regulate a wide array of processes essential for plant growth and development, including cell elongation, division, and differentiation. Auxin’s mode of action is uniquely characterized by its polar transport across cells and tissues, facilitated by a network of specialized transporters.
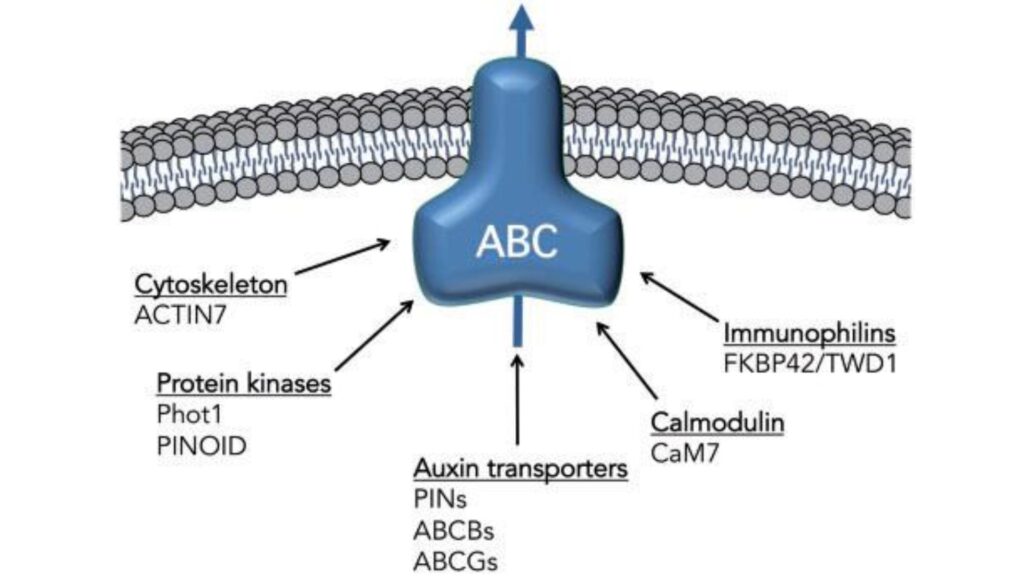
Among these, ATP-binding cassette subfamily B (ABCB) transporters play a key role in maintaining auxin homeostasis, predominantly by regulating its efflux. This regulation ensures the precise spatial and temporal distribution of auxin, which is critical for modulating various developmental processes (Geisler et al., 2014).
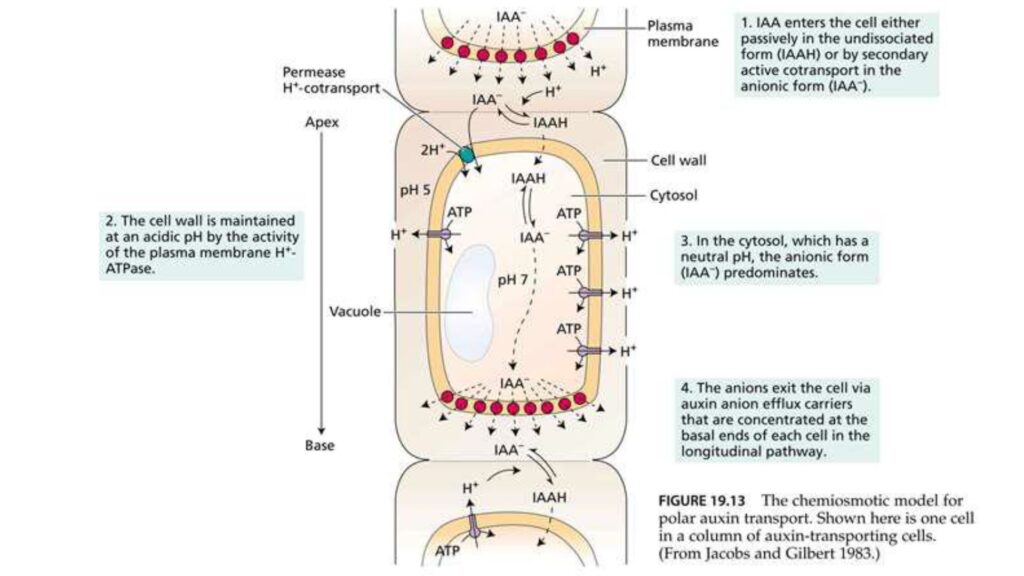
The chemiosmotic model describes polar auxin transport as a pH-dependent process. In the cytosol, where the pH is relatively high, auxin (indole-3-acetic acid, IAA) has reduced membrane permeability, necessitating active efflux through specialized carriers for directional export. In the acidic apoplast, auxin is protonated to its membrane-permeable form (IAAH), allowing passive diffusion into neighboring cells. This cycle, coupled with the asymmetric localization of efflux carriers, establishes the polarity of auxin transport, which is fundamental for plant growth and development.
Brassinosteroids (BRs), a class of plant steroid hormones, are detected at the plasma membrane by specific receptor kinases, such as BRASSINOSTEROID INSENSITIVE 1 (BRI1). Upon brassinosteroid binding, BRI1 undergoes activation, initiating a phosphorylation-dependent signaling cascade.
This cascade leads to the regulation of downstream transcription factors, ultimately influencing gene expression and a variety of physiological processes (Clouse and Sasse, 1998; Gudesblat and Russinova, 2011).
Additionally, brassinosteroid signaling exhibits significant crosstalk with auxin pathways, a critical interaction that establishes an integrated and dynamic regulatory network. This network modulates key growth and developmental responses, including cell elongation, division, and differentiation. The interplay between BR and auxin signaling highlights the complexity of hormonal interactions in plants and underscores the sophisticated mechanisms underlying their coordinated growth regulation (Vert et al., 2005; Wang et al., 2012).
Despite advances in understanding these pathways, a significant limitation persists in studying the dynamic regulation of ABCB transporter activity in response to fluctuating auxin and BR levels.
Traditional approaches fail to capture the real-time activity and regulatory mechanisms of these transporters under physiological conditions. Addressing this gap requires the development of innovative tools, such as activity sensors specifically designed for ABCB transporters.
Such sensors would enable real-time visualization and quantification of transporter activity, providing unprecedented insights into the interplay between auxin and BR signaling. This technological advancement could potentially transform our understanding of hormone transport mechanisms and their role in plant development.
LITERATURE REVIEW
Auxin and ABCB Transporters
Auxin, a pivotal plant hormone, regulates growth and development through its spatial distribution, which is tightly controlled by auxin transporters. These include PIN-FORMED (PIN) proteins and ATP-binding cassette subfamily B (ABCB) transporters. While PIN proteins mediate directional auxin transport within tissues due to their polar localization on the plasma membrane, ABCB transporters complement this role by facilitating long-distance auxin transport and maintaining cellular auxin homeostasis (Geisler et al., 2014; Petrášek & Friml, 2009).
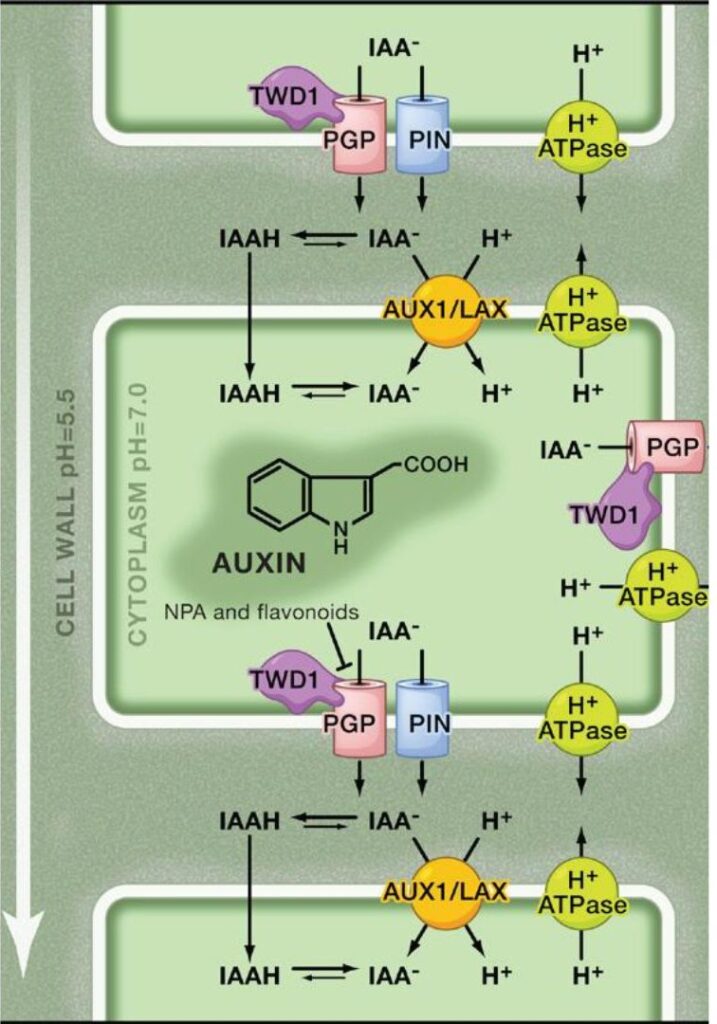
The apoplastic (cell wall) space maintains low pH via plasma membrane H⁺-ATPases, promoting protonation of indole-3-acetic acid (IAA). Protonated IAA (IAAH) diffuses passively into cells, while unprotonated IAA⁻ enters via H⁺/IAA⁻ symport through AUX1/LAX carriers. In the neutral cytosol, IAA deprotonates and becomes trapped. Auxin exits cells through PIN and ABCB (PGP) efflux carriers. ABCB transporter activity is modulated by NPA and flavonoids, which affect its interaction with the regulator TWD1. PIN protein polarity guides directional auxin flow across tissues (Vanneste & Friml, 2013).
Within the ABCB transporter family, ABCB1 and ABCB19 are well-characterized for their roles in long-distance auxin transport. These transporters localize to the plasma membrane and actively efflux auxin from cells, ensuring efficient distribution throughout the plant.
Such transport is crucial for various developmental processes, including organogenesis, vascular differentiation, and phototropic responses (Bailly et al., 2008). Mutations in ABCB19, for instance, disrupt auxin transport, leading to phenotypic anomalies such as impaired apical dominance and altered leaf morphology, highlighting its indispensable role in auxin-mediated growth regulation (Titapiwatanakun et al., 2009).
The activity of ABCB transporters is dynamically regulated by interacting proteins and post-translational modifications. TWISTED DWARF1 (TWD1), a FKBP42 immunophilin, interacts with ABCB1 and ABCB19 to stabilize their function, thereby enhancing auxin efflux efficiency (Bouchard et al., 2006).
Additionally, phosphorylation mediated by kinases such as those in the PINOID/WAG family influences the activity and localization of ABCBs, integrating their function into broader signaling networks responsive to hormonal and environmental stimuli.
Despite substantial advances, much of the knowledge about ABCB-mediated auxin transport has been derived from indirect methods, including radiolabeled auxin uptake assays, biochemical studies, and genetic analyses (Titapiwatanakun et al. 2009).
While these approaches have elucidated fundamental roles of ABCBs, they often lack the spatial and temporal resolution required to study transporter dynamics in vivo. For example, mutant analyses reveal phenotypic effects but do not directly capture transporter function or its regulatory responses to environmental changes.
Addressing these limitations requires the development of innovative tools capable of visualizing ABCB activity in real time. Advanced sensors tailored to ABCB transporters would enable high-resolution monitoring of auxin transport at cellular and subcellular levels. Such tools would provide critical insights into the coordination between ABCBs and PIN proteins, as well as the interplay between auxin and other hormonal pathways, including brassinosteroids and cytokinin.
These advancements could significantly enhance our understanding of auxin transport regulation and its implications for plant development and environmental adaptation (Geisler et al., 2014).
Brassinosteroids and Hormonal Crosstalk
Brassinosteroids (BRs) are a class of plant steroid hormones crucial for regulating various physiological processes, including cell expansion, vascular differentiation, and stress responses. These hormones exert their influence through a well-characterized signaling cascade that begins at the plasma membrane with the perception of BRs by the BRASSINOSTEROID INSENSITIVE 1 (BRI1) receptor kinase.
This cascade ultimately leads to the activation of transcription factors that control the expression of BR-responsive genes (Belkhadir et al., 2014). Beyond their direct roles, BRs are central players in hormonal crosstalk, interacting with other plant hormones such as auxin to fine-tune growth and development.
One of the most intriguing aspects of BR-auxin crosstalk is the modulation of auxin transport by BR signaling. Studies have demonstrated that BRs influence the localization and activity of auxin transporters, particularly the ATP-binding cassette subfamily B (ABCB) transporters.
For example, BR-activated signaling cascades have been shown to stabilize ABCB19 at the plasma membrane, ensuring efficient auxin efflux and long-distance transport (Belkhadir et al., 2014). This stabilization process appears to form a feedback loop in which BR signaling enhances auxin transport capacity, while auxin distribution influences BR-regulated developmental outcomes, such as cell elongation and vascular patterning.
The interplay between BR signaling and auxin transport is mediated by multiple mechanisms, including post-translational modifications of transporters and their associated proteins.
Phosphorylation of ABCB19, mediated by downstream components of the BR signaling pathway, may enhance its stability and functional activity at the plasma membrane. Additionally, BR signaling influences the trafficking of transporters, ensuring their optimal localization to sites where auxin transport is most needed.
These mechanisms underscore the sophisticated regulatory network that integrates BR and auxin pathways to coordinate plant development.
While significant progress has been made in elucidating the interaction between BR and auxin pathways, much of the evidence relies on indirect biochemical assays, genetic analyses, and mutant studies.
For example, immunoblotting and protein localization studies have been instrumental in revealing the effects of BR signaling on ABCB19 stability. However, these approaches are limited in their ability to provide real-time, spatially resolved insights into transporter dynamics under physiological conditions.
Moreover, biochemical assays often fail to capture the complexity of in vivo interactions, such as transient signaling events or the influence of environmental factors on hormonal crosstalk.
To overcome these limitations, the development of sophisticated tools for in vivo studies is essential. Fluorescently tagged sensors for BR signaling components and auxin transporters could enable high-resolution visualization of their dynamics in real-time.
Additionally, advanced imaging techniques such as Förster resonance energy transfer (FRET)-based sensors and single-molecule tracking could provide deeper insights into how BR signaling modulates auxin transport at the molecular level.
These innovations would not only validate current models of BR-auxin crosstalk but also uncover new layers of regulation, enhancing our understanding of how plants integrate multiple hormonal signals to optimize growth and development.
Advances in Sensor Development
The study of plant hormone dynamics has been significantly advanced by the development of biosensors, which enable precise, real-time visualization of molecular processes in living cells. Among these, Förster Resonance Energy Transfer (FRET)-based sensors have been instrumental in studying plant hormones and their signaling pathways.
These sensors rely on energy transfer between two fluorescent proteins, typically a donor and an acceptor, whose efficiency changes in response to molecular interactions or conformational changes. FRET-based sensors have been successfully applied to monitor auxin concentrations, kinase activities, and other signaling components, providing unprecedented insights into their spatial and temporal regulation (Jones et al., 2014; Friml & Jones, 2010).
Genetically encoded sensors represent a significant leap forward in plant hormone research. These sensors are designed to operate within living cells, enabling live-cell imaging and real-time analysis of molecular dynamics.
For example, the development of auxin sensors such as DII-VENUS has allowed researchers to map auxin gradients and monitor auxin responses at cellular and subcellular resolutions (Vanneste & Friml, 2013). Similarly, sensors targeting specific kinases involved in hormone signaling have provided detailed insights into the activation and regulation of signaling cascades (Belkhadir et al., 2014). These tools have bridged a critical gap between biochemical assays and in vivo observations, offering a more dynamic understanding of hormone biology.
Despite these advancements, the field still lacks dedicated sensors for monitoring the activity of ABCB transporters. ABCB transporters, particularly ABCB1 and ABCB19, play vital roles in auxin efflux and are key targets for regulation by brassinosteroid (BR) signaling pathways (Geisler et al., 2017; Titapiwatanakun et al., 2009). However, the dynamic behavior of these transporters
and their regulatory responses to hormonal cues remain poorly understood. Current methodologies, such as radiolabeled auxin uptake assays and protein localization studies, provide indirect and often static snapshots of transporter function, failing to capture the real-time dynamics of auxin efflux or BR-mediated regulation (Bouchard et al., 2006).
Developing sensors tailored to ABCB transporters could transform the study of auxin transport and hormonal crosstalk. Such sensors would need to be designed to detect the conformational changes or activity states of ABCB transporters in real time. For example, FRET-based approaches could be adapted to monitor changes in ABCB transporter activity by tagging them with fluorescent probes sensitive to auxin binding or transport states. Similarly, biosensors could be designed to detect BR-mediated modifications, such as phosphorylation events, which influence ABCB activity and localization (Kim & Wang, 2010).
The creation of ABCB-specific sensors would address several critical gaps in plant hormone research. These sensors could provide high-resolution insights into how auxin efflux is regulated at subcellular levels, how ABCB transporters interact with other components like PIN proteins (Petrášek & Friml, 2009), and how BR signaling influences these processes (Belkhadir et al., 2014; Wang et al., 2002).
Moreover, these tools would facilitate the study of dynamic responses to environmental cues, enhancing our understanding of how plants adapt to changing conditions. By combining these sensors with advanced imaging techniques, such as confocal or super-resolution microscopy, researchers could gain a comprehensive view of auxin transport and its regulation in vivo.
RESEARCH GAP
While ABCB transporters’ roles in auxin efflux and BR-mediated crosstalk are well-established, the absence of precise, real-time activity sensors limits our ability to:
1. Quantify dynamic changes in ABCB activity in vivo.
2. Decipher spatiotemporal regulation of auxin transport by BRs.
3. Investigate ABCB regulation under environmental and developmental cues.
HYPOTHESIS
Genetically encoded ABCB-type activity sensors can elucidate the spatiotemporal dynamics of auxin efflux and its modulation by BR signaling, thereby providing critical insights into hormone cross-talk and transporter regulation.
OBJECTIVES
1.Develop and validate FRET-based ABCB activity sensors.
2.Investigate spatiotemporal dynamics of ABCB-mediated auxin transport in response to BR signaling.
3.Apply the sensors to study ABCB regulation under various environmental and developmental contexts.
MATERIALS AND METHODS
a). Sensor Design
In the development of advanced sensor systems, a key focus lies on sensor design, particularly through the utilization of genetic constructs and expression systems. For this purpose, fluorescence resonance energy transfer (FRET)-based sensors are designed by incorporating sequences from ABCB transporters fused with FRET donor and acceptor fluorophores, such as mCitrine and mCerulean, enabling precise detection of molecular interactions or changes.
These constructs are then cloned into appropriate expression vectors tailored for transformation into Arabidopsis thaliana. This approach facilitates the in vivo study of transporter activity and enables high-resolution imaging of dynamic cellular processes (Bouchard et al., 2006; Geisler et al., 2017).
b). Validation of Sensor Activity
Validating the activity of FRET-based sensors is a fundamental step in confirming their accuracy and functionality for studying auxin transport. This validation employs a multi-faceted approach to ensure the sensors perform effectively in diverse contexts.
The first stage involves in vitro assays where the sensors are expressed in yeast systems. This controlled setup allows researchers to test the sensors’ capacity to monitor auxin transport activity without the complexity of plant systems, providing a baseline evaluation of their performance (Petrášek & Friml, 2009).
Following in vitro validation, live-cell imaging is conducted using confocal microscopy to measure FRET efficiency directly within Arabidopsis protoplasts. This step enables the observation of sensor activity in a cellular environment, offering a closer approximation to the in vivo conditions in which the sensors will be used. The real-time imaging not only confirms sensor functionality but also provides valuable spatial and temporal data on auxin dynamics within cells (Jones et al., 2014).
To further corroborate the accuracy of the sensors, biochemical correlation studies are performed. These studies compare FRET readouts with results from radiolabeled auxin transport assays, a well-established method for quantifying auxin movement. By aligning the FRET signals with biochemical transport data, researchers can validate that the sensor output reliably reflects physiological auxin transport processes (Titapiwatanakun et al., 2009).
c). Application in BR-Auxin Crosstalk Studies
In studies investigating the crosstalk between brassinosteroids (BRs) and auxin, researchers employ a multifaceted approach to elucidate regulatory mechanisms. BR treatments involve the application of BR analogs (e.g., brassinolide) and inhibitors (e.g., brassinazole) to Arabidopsis thaliana expressing auxin sensors, enabling the manipulation of BR signaling pathways to observe downstream effects on auxin dynamics (Clouse, 2011).
Dynamic imaging techniques, such as Förster Resonance Energy Transfer (FRET)-based live-cell imaging, are utilized to monitor real-time auxin efflux changes in root and shoot tissues, providing spatial and temporal resolution of auxin transport under BR modulation (Friml & Jones, 2010).
To dissect the molecular basis of BR-auxin interactions, genetic disruptions—including mutations in key BR signaling components like BRI1 (brassinosteroid-insensitive 1) or BIN2 (brassinosteroid-insensitive 2)—are introduced to assess their impact on ATP-binding cassette subfamily B (ABCB) transporter activity, which governs auxin efflux (Kim & Wang, 2010).
Together, these methodologies reveal how BR signaling pathways transcriptionally and post-translationally regulate ABCB transporters, offering insights into the hormonal crosstalk critical for plant growth and development.
d). Environmental and Developmental Contexts
The study of ABCB transporter activity in varying environmental and developmental contexts provides critical insights into their role in plant adaptation and growth.
In the context of abiotic stress experiments, plants are exposed to stressors such as salinity and drought, which mimic challenging environmental conditions. By monitoring ABCB activity under these stresses, researchers can identify dynamic changes in transporter function, potentially revealing mechanisms by which plants regulate auxin transport to maintain growth and stress resilience.
These observations can help elucidate how ABCB transporters contribute to stress signaling pathways and resource allocation under adverse conditions (Geisler et al., 2017; Vanneste & Friml, 2013).
In parallel, the role of ABCB transporters during plant development is examined by tracking their activity across different stages of morphogenesis, particularly in roots and shoots. During root development, ABCB transporters influence auxin gradients that dictate root elongation, branching, and directional growth (Reemmer & Murphy, 2014).
Similarly, in shoots, these transporters are involved in maintaining auxin homeostasis, critical for shoot apical dominance, leaf formation, and overall plant architecture (Titapiwatanakun et al., 2009).
By investigating ABCB transporter dynamics throughout these developmental stages, researchers can better understand how plants integrate environmental cues and developmental signals to regulate growth and adapt to changing conditions.
WORK PLAN
Year 1: Sensor Design and Initial Validation
•Design FRET-based ABCB activity sensors, incorporating appropriate donor and acceptor fluorophores. Clone constructs into yeast expression systems and validate their auxin transport activity.
•Introduce sensor constructs into Arabidopsis protoplasts for preliminary imaging studies. Perform radiolabeled auxin uptake assays to establish a biochemical correlation.
Year 2: Advanced Validation and BR-Auxin Crosstalk Studies
•Conduct comprehensive imaging of auxin transport dynamics in whole Arabidopsis plants. Treat sensor-expressing plants with BR analogs and inhibitors to evaluate the impact on ABCB activity. Analyze FRET efficiency in BR signaling mutants.
•Extend studies to include the effects of BR signaling on ABCB transporter localization and activity under varying environmental conditions, such as light and temperature.
Year 3: Environmental and Developmental Contextual Studies
•Investigate the role of ABCB transporters in response to abiotic stresses (e.g., salinity, drought). Study transporter activity during key developmental processes such as root and shoot elongation. Compile and analyze the generated data to identify patterns and mechanistic insights.
Year 4: Data Analysis and Dissertation Writing
•Perform in-depth statistical analyses and integrate findings into a cohesive narrative. Prepare manuscripts for peer-reviewed publications. Write the dissertation, incorporating all experimental results, and defend the research work successfully.
REFERENCES
1.Bailly, A., et al. (2008). Modulation of P-glycoproteins by Auxin Transport Inhibitors Is Mediated by Interaction with Immunophilins. Journal of Biological Chemistry, 283(31), 21817 – 21826.
2.Belkhadir, Y., et al. (2014). The molecular circuitry of brassinosteroid signaling. New phytologist, 206(2):522-540.
3.Belkhadir, Y., Jaillais, Y., Epple, P., Balsemão-Pires, E., Dangl, J. L., & Chory, J. (2014). Brassinosteroids modulate the efficiency of plant immune responses to microbe-associated molecular patterns. Proceedings of the National Academy of Sciences, 111(20), 761-770.
4.Bouchard, R., et al. (2006). Immunophilin-like TWISTED DWARF1 modulates ABCB-mediated auxin transport in Arabidopsis. Journal of Biological Chemistry, 281(41), 30603 – 30612.
5. Clouse, S. D. (2011). Brassinosteroid signal transduction: From receptor kinase activation to transcriptional networks regulating plant development. The Plant Cell, *23*(4), 1219–1230.
6.Clouse, S. D., & Sasse, J. M. (1998). Brassinosteroids: Essential regulators of plant growth and development. Annual Review of Plant Physiology and Plant Molecular Biology, 49(1), 427–451.
7. Friml, J., & Jones, A. R. (2010). Endoplasmic reticulum: The rising compartment in auxin biology. Plant Physiology, *154*(2), 458–462.
8.Geisler, M., et al. (2017). A Critical View on ABC Transporters and Their Interacting Partners in Auxin Transport.Plant and Cell Physiology.
9.Geisler, M., Wang, B., & Zhu, J. (2014). Auxin transport during root gravitropism: A new role for ABCB transporters. Plant Signaling & Behavior, 9(12), e972843.
10.Gudesblat, G. E., & Russinova, E. (2011). Plants grow on brassinosteroids. Current Opinion in Plant Biology, 14(5), 530–537.
11. Jones, A.M., et al. (2014). Abscisic acid dynamics in roots detected with genetically encoded FRET sensors. eLife 3: e01741.
12. Kim, T.-W., & Wang, Z.-Y. (2010). Brassinosteroid Signal Transduction from Receptor Kinases to Transcription Factors. Annual Review of Plant Biology, 61:681-704.
13.Petrášek, J., & Friml, J. (2009). Auxin transport routes in plant development. Development, 136(16), 2675–2688.
14.Reemmer, J., & Murphy, A. (2014). Intercellular Transport of Auxin. In: Zažímalová, E., Petrášek, J., Benková, E. (eds) Auxin and Its Role in Plant Development. Springer, Vienna.
15. Titapiwatanakun, B., et al. (2009). ABCB19/PGP19 stabilises PIN1 in membrane microdomains in Arabidopsis. The plant Journal.
16.Vanneste, S., & Friml, J. (2013). Auxin: A trigger for change in plant development. Cell, 153(3), 510-520.
17.Vert, G., et al. (2008). Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proceedings of the National Academy of Sciences, 105 (28) 9829-9834.
18.Wang, Z. Y., et al. (2002). Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Developmental Cell, 2(4), 505–513.
